Multiple Choice
Predict the products by completing a balanced equation for the following decomposition reaction. CaCl2(l) 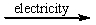 ?
?
A) CaCl2(l) 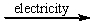 Ca(l) + 2Cl-(l)
Ca(l) + 2Cl-(l)
B) CaCl2(l) 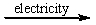 Ca2+(l) + Cl2(g)
Ca2+(l) + Cl2(g)
C) CaCl2(l) 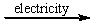 Ca2+(l) + 2Cl-(l)
Ca2+(l) + 2Cl-(l)
D) CaCl2(l) 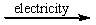 Ca(l) + Cl2(g)
Ca(l) + Cl2(g)
E) CaCl2(l) 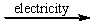 CaCl(l) + Cl-(l)
CaCl(l) + Cl-(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q30: A particular reaction may be both a
Q51: A 0.150 M sodium chloride solution is
Q54: In an acid-base (neutralization) reaction the indicator
Q60: Select the classification for the following reaction.
Q61: Which one of the following is not
Q62: Select the classification for the following reaction.
Q64: Select the classification for the following reaction.
Q66: Write down the oxidation number of the
Q82: Which of the following is a strong
Q106: How many moles of H<sup>+</sup>(aq) ions are