Short Answer
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its absolute temperature, other factors remaining constant? 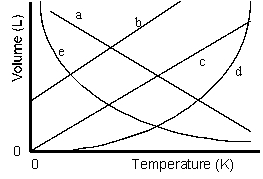
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: A sample of carbon dioxide gas at
Q16: A 500-mL sample of argon at 800
Q39: A gas mixture, with a total pressure
Q40: A gas consists of 85.7 % carbon
Q43: Aluminum metal shavings (10.0 g) are placed
Q48: A flask containing neon gas is connected
Q49: State Avogadro's Law.
Q53: A weather balloon was initially at a
Q83: What is the pressure in a 7.50-L
Q93: For an ideal gas, a plot of