Short Answer
In the quantum mechanical treatment of the hydrogen atom, which one of the following combinations of quantum numbers is not allowed? 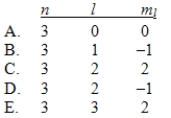
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Use the Rydberg equation to calculate the
Q10: An electron in the n = 6
Q36: In the Bohr model of the hydrogen
Q51: Who proposed the principle which states that
Q53: The following combinations of quantum numbers are
Q56: a. Calculate the momentum of a photon
Q57: Platinum, which is widely used as a
Q59: Use the Bohr equation to calculate the
Q62: A modern compact fluorescent lamp contains 1.4
Q68: Which one of the following sets of