Multiple Choice
Select the correct set of quantum numbers (n, l, ml, ms) for the highest energy electron in the ground state of tin, Sn.
A) 5, 2, -1, 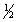
B) 5, 2, 0, 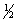
C) 5, 1, 2, 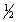
D) 5, 1, 0, 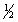
E) 5, 2, 1, 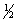
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Which of the following electron configurations is
Q12: Metallic behavior is generally associated with<br>A)elements with
Q23: Select the most acidic compound from the
Q24: Select the correct electron configuration for sulfur
Q30: How many valence electrons are there in
Q43: Select the element with the most negative
Q51: Which of the following elements has the
Q72: Which one of the following equations correctly
Q74: Elements with _ first ionization energies and
Q82: Write down the maximum number of electrons