Not Answered
Calculate the lattice energy of magnesium sulfide from the data given below.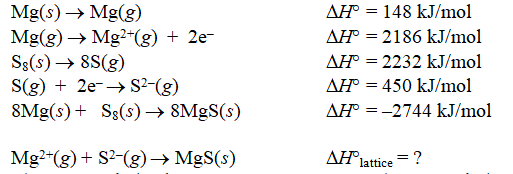
A) -3406 kJ/mol
B) -2720. kJ/mol
C) 2720. kJ/mol
D)3406 kJ/mol
E)none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The lattice energy of large ions is
Q10: For which of the following elements (in
Q26: Bond energy increases as bond order increases,
Q33: Based on electronegativity trends in the periodic
Q35: Covalently bonded substances do not necessarily exist
Q36: Electronegativity is a measure of<br>A)the energy needed
Q39: Select the element whose Lewis symbol is
Q43: Select the strongest bond in the following
Q44: Combustion of a fat will release more
Q53: Which one of the following properties is