Multiple Choice
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ) the standard enthalpy change H° for the hydrogenation of ethyne (acetylene) to ethane. 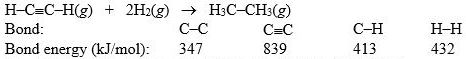
A) -296 kJ
B) -51 kJ
C) 51 kJ
D) 296 kJ
E) 381 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Select the most polar bond amongst the
Q16: Which of the following is a covalent
Q17: Which of the following elements is the
Q25: Which of the following elements is the
Q40: The electrostatic energy of two charged particles
Q47: Which of the following period 3 chlorides
Q54: The melting points of metals are only
Q64: Ionic bonding typically occurs when a _
Q67: The lattice energy for ionic crystals increases
Q74: When an atom is represented in a