Multiple Choice
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given. 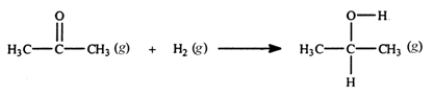

A) -484 kJ
B) -366 kJ
C) -48 kJ
D) +48 kJ
E) +366 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Select the compound with the lowest (i.e.,
Q7: Give a clear and concise definition of
Q12: Which of the following compounds displays the
Q14: The lattice energy of MgCl<sub>2</sub> is the
Q15: When one mole of each of the
Q18: A single covalent bond consists of a
Q24: As a measure of the strength of
Q31: The stronger the bonds in a fuel,
Q48: Electronegativities on Pauling's scale are calculated from
Q50: Quartz (SiO<sub>2</sub>) is a solid with a