Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of ____ and an oxidation number of ____. 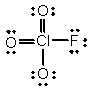
A) 7, 7
B) 7, -1
C) 1, 1
D) 1, -1
E) 1, 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Select the correct Lewis structure for TeBr<sub>2</sub>.<br>A)
Q33: Select the Lewis structure for XeO<sub>2</sub>F<sub>2 </sub>which
Q35: Which one of the following Lewis structures
Q36: What is the molecular shape of XeO<sub>2</sub>F<sub>2
Q38: a. Draw and name three molecular shapes
Q39: Oxygen difluoride is a powerful oxidizing and
Q56: In which one of the following molecules
Q60: Predict the ideal bond angles in FNO
Q76: What is the molecular shape of ClF<sub>2</sub><sup>-</sup>
Q97: Predict the smallest actual bond angle in