Multiple Choice
In the following Lewis structure for phosphate, phosphorus has a formal charge of ____ and an oxidation number of ____. 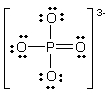
A) 0, -3
B) 0, 5
C) 5, -3
D) 5, 5
E) 3, 5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Draw Lewis structures, showing all valence electrons,
Q14: Which one of the following Lewis structures
Q18: A molecule which contains polar bonds will
Q21: Draw Lewis structures which obey the octet
Q33: In which one of the following is
Q61: Use VSEPR theory to decide which one
Q63: List all possible molecular geometries (shapes) for
Q66: Use VSEPR theory to decide which one
Q76: Considering all the bonds in a molecule
Q88: Which of the following has no net