Multiple Choice
The formal charge on Cl in the structure shown for the perchlorate ion is 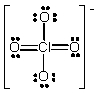
A) -2
B) -1
C) 0
D) +1
E) +2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: Which one of the following Lewis structures
Q36: What is the molecular shape of XeO<sub>2</sub>F<sub>2
Q38: a. Draw and name three molecular shapes
Q39: Oxygen difluoride is a powerful oxidizing and
Q41: Thionyl chloride is used as an oxidizing
Q42: The formal charges on Cl and O
Q43: Which one of the following Lewis structures
Q44: In which one of the following structures
Q55: According to VSEPR theory, a molecule with
Q76: What is the molecular shape of ClF<sub>2</sub><sup>-</sup>