Essay
The Lewis structure of formaldehyde, CH2O, is shown. Use VSEPR theory to predict the molecular geometry and the H-C-H bond angle. Outline your reasoning. 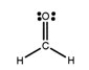
Correct Answer:

Verified
There are three electron groups around t...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Which one of the following molecules has
Q11: When resonance occurs, the bond lengths in
Q19: What is the molecular shape of NO<sub>2</sub><sup>-</sup>
Q35: According to VSEPR theory, a molecule with
Q54: In neutral molecules, how many bonds are
Q62: According to VSEPR theory, a molecule with
Q70: According to VSEPR theory, a molecule with
Q99: In which of the following does the
Q101: Draw the Lewis structure of XeF<sub>4</sub>. Use
Q108: Select the best Lewis structure for P<sub>2</sub>I<sub>4</sub>.<br>A)