Multiple Choice
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. 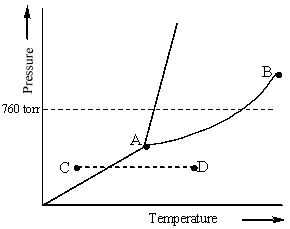
A) Bo(s) has a lower density than Bo(l) .
B) The triple point for Bo is at a higher temperature than the melting point for Bo.
C) Bo changes from a solid to a liquid as one follows the line from C to D.
D) Bo changes from a liquid to a gas as one follows the line from C to D.
E) Point B represents the critical temperature and pressure for Bo.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Crystal structures may be conveniently measured using<br>A)X-ray
Q31: The Clausius-Clapeyron equation is used in calculations
Q68: a. Explain what is meant by the
Q70: Which of the following has a boiling
Q71: Select the pair of substances in which
Q73: Strontium metal crystallizes in a cubic unit
Q74: Assuming that atoms are spherical, calculate the
Q75: Examine the following phase diagram and determine
Q88: Which of the following should have the
Q95: Octane is a component of fuel used