Multiple Choice
Consider the following phase diagram and identify the process occurring as one goes from point C to point D. 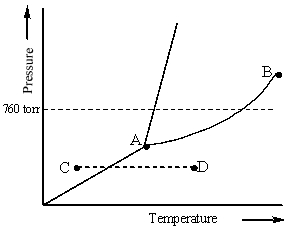
A) increasing temperature with a phase change from solid to liquid
B) increasing temperature with a phase change from solid to vapor
C) increasing temperature with a phase change from liquid to vapor
D) increasing temperature with no phase change
E) increasing temperature beyond the critical point
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Octane has a vapor pressure of 40.
Q30: Only molecules which do not have dipole
Q31: Draw a fully labeled phase diagram (P
Q33: Iron has a body-centered cubic unit cell,
Q34: Liquid ammonia boils at -33.4°C and has
Q35: The energy gap between the conduction band
Q37: The density of solid sodium chloride, NaCl,
Q39: A temperature increase causes _ in the
Q46: All gases can be liquefied at room
Q82: The surface tension of water is lowered