Essay
Consider the phase diagram shown below. 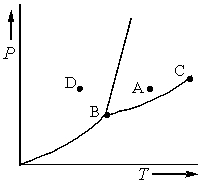 a. What phase(s) is/are present at point A?
a. What phase(s) is/are present at point A?
b. What phase(s) is/are present at point B?
c. Name point C and explain its significance.
d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen.
Correct Answer:

Verified
a. liquid
b. solid, liquid, and gas
c. C...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
b. solid, liquid, and gas
c. C...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q9: The phase diagram of a substance can
Q21: A certain solid metallic element has a
Q29: Some of the information obtained from the
Q38: Which one of the following quantities is
Q54: In an ionic solid MX consisting of
Q55: Assuming that atoms are spherical, calculate the
Q55: For the solid forms of the following
Q56: What adjective best describes the solid compound
Q57: Which of the following pairs is arranged
Q57: When liquid bromine is cooled to form