Multiple Choice
Saccharin, one of the first non-nutritive sweeteners used in soft-drinks, is 500 times sweeter than sugar in dilute aqueous solutions. The solubility of saccharin is 1.00 gram per 290 mL of solution. What is the molarity of a saturated saccharin solution? ( 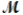 saccharin = 183.2 g/mol)
saccharin = 183.2 g/mol)
A) 0.0188 M
B) 0.632 M
C) 1.58 M
D) 3.45 M
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Two aqueous are prepared: 1.00 m Na<sub>2</sub>CO<sub>3
Q6: Calculate the vapor pressure of a solution
Q8: Cinnamaldehyde ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="Cinnamaldehyde (
Q9: Carbon tetrachloride, once widely used in fire
Q10: Procaine hydrochloride ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="Procaine hydrochloride
Q20: A solution of ethanol (C<sub>2</sub>H<sub>6</sub>O) in water
Q38: Ideal solutions do not conform to Raoult's
Q40: From the following list of aqueous solutions
Q48: What concentration of aqueous FeCl<sub>3</sub> would have
Q73: The most abundant molecules in the cell