Multiple Choice
Which of the following statements describes the correct method of preparation of 1.00 L of a 2.0 M urea solution? 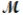 urea = 60.06 g/mol
urea = 60.06 g/mol
A) Dissolve 120 g of urea in 1.00 kg of distilled water.
B) Dissolve 120 g of urea in 880 g of distilled water.
C) Dissolve 120 g of urea in enough distilled water to produce 1.00 L of solution.
D) Dissolve 120 g of urea in 1.00 liter of distilled water.
E) The density of urea is needed in order to do this calculation.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: An emulsion is a dispersion consisting of
Q9: Raoult's Law relates the vapor pressure of
Q12: Select the weakest electrolyte from the following
Q14: A 0.89% (w/v) sodium chloride solution is
Q18: Which of the following pairs of ions
Q19: Select the type of interaction which best
Q23: Safrole is used as a topical antiseptic.
Q24: If a 100-mL sample of a volatile
Q52: The concentration of iodine in sea water
Q74: The solubility of gases in water increases