Multiple Choice
Barbiturates are synthetic drugs used as sedatives and hypnotics. Barbital ( 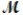 = 184.2 g/mol) is one of the simplest of these drugs. What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid? Kb = 3.07°C/m; boiling point of pure acetic acid = 117.9°C
= 184.2 g/mol) is one of the simplest of these drugs. What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid? Kb = 3.07°C/m; boiling point of pure acetic acid = 117.9°C
A) 117.0°C
B) 117.7°C
C) 118.1°C
D) 118.8°C
E) >120°C
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Which of the following aqueous solutions should
Q17: Soda drinks bubble when the bottle is
Q21: What volume of concentrated (14.7 M) phosphoric
Q25: The mole fraction of potassium nitrate in
Q57: Which of the following ions will be
Q58: Raoult's Law relates the vapor pressure of
Q59: The larger the ion, the greater in
Q59: Classify the following as either solutions or
Q66: A 7.112 M solution of sulfuric acid
Q71: A solution of sucrose (sugar) in water