Multiple Choice
Hexachlorophene is used as a disinfectant in germicidal soaps. What mass of hexachlorophene ( 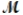 = 406.9 g/mol) must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C? Kb = 3.63°C/m, boiling point of pure chloroform = 61.70°C
= 406.9 g/mol) must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C? Kb = 3.63°C/m, boiling point of pure chloroform = 61.70°C
A) 12.6 g
B) 17.2 g
C) 31.0 g
D) 34.4 g
E) 101 g
Correct Answer:

Verified
Correct Answer:
Verified
Q6: A 0.100 m K<sub>2</sub>SO<sub>4</sub> solution has a
Q26: The solubility of the oxidizing agent potassium
Q34: Which of the following aqueous solutions will
Q70: The vapor pressure of pure acetone (propanone)
Q94: If the shell of a raw egg
Q96: Determine the freezing point of a solution
Q97: How many moles of solute particles are
Q101: For a given solution, which of the
Q102: Diethyl ether has a vapor pressure of
Q104: What is the molality of a solution