Essay
In the gas phase at 500.°C, cyclopropane reacts to form propene in a first-order reaction. The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L) plotted versus time. 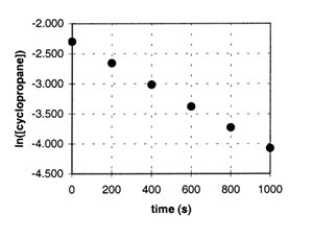 a. Explain how this plot confirms that the reaction is first order.
a. Explain how this plot confirms that the reaction is first order.
b. Calculate the first-order rate constant, k.
c. Determine the initial concentration of
cyclopropane in this experiment.
Correct Answer:

Verified
a. The fact that a plot of ln ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: You are required to determine the energy
Q4: Which one of the following sets of
Q6: Cyclopropane is converted to propene in a
Q8: For the reaction A(g) + 2B(g) <font
Q9: A reaction has an activation energy of
Q11: The greater the energy of activation, E<sub>a</sub>,
Q12: A rate constant obeys the Arrhenius equation,
Q25: A reaction intermediate is a species corresponding
Q40: Which of the following affects the activation
Q61: An elementary reaction is a simple, one-step