Essay
Cyclobutane decomposes to ethene in a first-order reaction. From measurements of the rate constant (k) at various absolute temperatures (T), the accompanying Arrhenius plot was obtained (ln k versus 1/T). 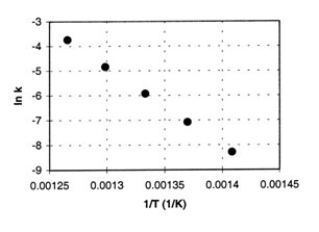 a. Calculate the energy of activation, Ea.
a. Calculate the energy of activation, Ea.
b. Determine the value of the rate constant at 740. K. (In the plot, the units of k are s-1.)
Correct Answer:

Verified
a. 260 ± 2...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: A catalyst accelerates a reaction because<br>A)it increases
Q9: The units of the rate of reaction
Q31: In going from room temperature (25.0°C) to
Q62: For the reaction 3A(g) + 2B(g) <font
Q64: The compound RX<sub>3</sub> decomposes according to the
Q68: Briefly list the features/properties common to all
Q70: A gas-phase decomposition is first-order with respect
Q70: The decomposition of dinitrogen pentaoxide has an
Q71: The rate constant for a reaction is
Q72: In the collision theory of reaction rates,