Multiple Choice
What is the mass-action expression, Qc , for the following chemical reaction? PbO(s) + CO(g)  Pb(l) + CO2(g)
Pb(l) + CO2(g)
A) 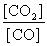
B) 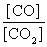
C) 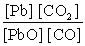
D) 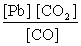
E) None of the above expressions is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q66: When a chemical system is at equilibrium,<br>A)the
Q94: At a certain temperature the reaction CO<sub>2</sub>(g)
Q95: A mixture 0.500 mole of carbon monoxide
Q96: Nitrogen dioxide can dissociate to nitric oxide
Q97: Write the mass-action expression, Q<sub>c</sub>, for the
Q98: Consider the following gas-phase equilibrium reaction:<br>N<sub>2</sub>(g) +
Q99: A chemical reaction has an equilibrium constant
Q100: The equilibrium constant K<sub>c</sub> for the reaction
Q101: What is the mass-action expression, Q<sub>c </sub>,
Q104: Carbon monoxide and chlorine combine in an