Multiple Choice
Write the mass-action expression, Qc , for the following chemical reaction. Sn2+(aq) + 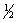 O2(g) + 3H2O(l)
O2(g) + 3H2O(l)  SnO2(s) + 2H3O+(aq)
SnO2(s) + 2H3O+(aq)
A) 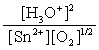
B) 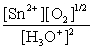
C) 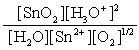
D) 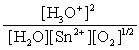
E) None of the above expressions is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: For a solution equilibrium, a change in
Q64: At 500°C the equilibrium constant, K<sub>p </sub>,
Q65: Write the expressions for K<sub>c</sub> and K<sub>p</sub>
Q66: Nitric oxide is formed in automobile exhaust
Q67: H<sub>2</sub>SO<sub>3</sub>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="H<sub>2</sub>SO<sub>3</sub>(aq) <sub>
Q68: Consider the reactions of cadmium with the
Q70: What is the mass-action expression, Q<sub>c</sub> ,
Q71: The reaction of nitric oxide to form
Q72: The equilibrium constant, K<sub>c </sub>, for the
Q73: An equilibrium is established in which both