Multiple Choice
The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 58.0 at a certain temperature. Br2(g) + Cl2(g)  2BrCl(g)
2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g) 
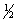 Br2(g) +
Br2(g) + 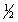 Cl2(g)
Cl2(g)
A) 2.97 × 10-4
B) 1.72 × 10-2
C) 3.45 × 10-2
D) 1.31 × 10-1
E) > 1.00
Correct Answer:

Verified
Correct Answer:
Verified
Q16: For a gas-phase equilibrium, a change in
Q46: Changing the amount of a solid reactant
Q80: Ammonium iodide dissociates reversibly to ammonia and
Q81: Magnesium hydroxide is used in several antacid
Q82: The equilibrium constant, K<sub>p </sub>, for the
Q83: Compounds A, B, and C react according
Q84: Hydrogen sulfide will react with water as
Q88: The reaction quotient, Q<sub>c</sub>, for a reaction
Q89: Nitrogen dioxide decomposes according to the reaction
Q90: Consider the equilibrium reaction shown below. B<sub>2</sub>(g)