Multiple Choice
Hydrogen sulfide can be formed in the following reaction: H2(g) + 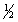 S2(g)
S2(g)  H2S(g) H°rxn = -92 kJ
H2S(g) H°rxn = -92 kJ
The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598
Correct Answer:

Verified
Correct Answer:
Verified
Q16: For a gas-phase equilibrium, a change in
Q46: Changing the amount of a solid reactant
Q88: The reaction quotient, Q<sub>c</sub>, for a reaction
Q89: Nitrogen dioxide decomposes according to the reaction
Q90: Consider the equilibrium reaction shown below. B<sub>2</sub>(g)
Q92: Consider the equilibrium reaction: N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg"
Q93: The following reaction is at equilibrium at
Q94: At a certain temperature the reaction CO<sub>2</sub>(g)
Q95: A mixture 0.500 mole of carbon monoxide
Q96: Nitrogen dioxide can dissociate to nitric oxide