Multiple Choice
Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak base (0.10 mol L-1) with HCl of the same concentration?
A) 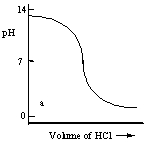
B) 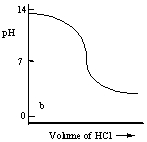
C) 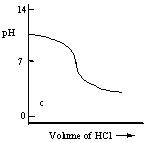
D) 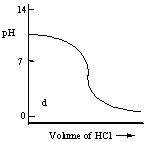
E) 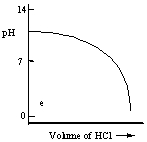
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Calculate the solubility of magnesium sulfate, MgSO<sub>4</sub>,
Q51: For a diprotic acid H<sub>2</sub>A, the relationship
Q52: The solubility of calcium chromate is 1.56
Q95: Buffer solutions with the component concentrations shown
Q96: Calculate the solubility of copper(II) carbonate, CuCO<sub>3</sub>,
Q97: A change in pH will significantly affect
Q98: Use the following information to calculate the
Q99: You need to prepare a buffer solution
Q103: Make a clear distinction between buffer range
Q106: When a weak acid is titrated with