Multiple Choice
Write the ion product expression for magnesium fluoride, MgF2.
A) 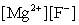
B) 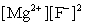
C) 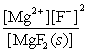
D) 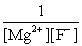
E) 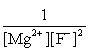
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The end point in a titration is
Q33: Increasing the concentrations of the components of
Q38: A solution is prepared by mixing 50.0
Q73: Equal volumes of the following pairs of
Q86: A popular buffer solution consists of carbonate
Q86: A buffer is prepared by adding 0.5
Q91: A buffer is prepared by adding 1.00
Q99: What mass of NaF must be added
Q104: A 10.0-mL sample of 0.75 M CH<sub>3</sub>CH<sub>2</sub>COOH
Q111: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution