Multiple Choice
Write the ion product expression for silver sulfide, Ag2S.
A) 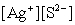
B) 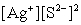
C) 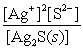
D) 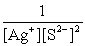
E) 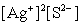
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Barium sulfate (BaSO<sub>4</sub>) is a slightly soluble
Q12: Which, if any, of the following aqueous
Q13: Fe(NO<sub>3</sub>)<sub>3</sub> (0.00100 mol) and KSCN (0.200 mol)
Q15: A 35.0-mL sample of 0.20 M LiOH
Q17: Lead(II) iodide, PbI<sub>2</sub>, is an ionic compound
Q19: The salts X(NO<sub>3</sub>)<sub>2 </sub>and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q45: A 20.0-mL sample of 0.30 M HBr
Q60: Increasing the concentrations of the components of
Q62: Calculate the solubility of silver oxalate, Ag<sub>2</sub>C<sub>2</sub>O<sub>4</sub>,
Q97: A phosphate buffer (H<sub>2</sub>PO<sub>4</sub><sup>-</sup>/HPO<sub>4</sub><sup>2-</sup>) has a pH