Multiple Choice
Consider the dissolution of MnS in water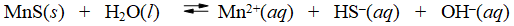
How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
A) The solubility will be unchanged.
B) The solubility will decrease.
C) The solubility will increase.
D) The amount of KOH added must be known before its effect can be predicted.
E) The pKa of H2S is needed before a reliable prediction can be made.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: When a strong acid is titrated with
Q32: A lab technician adds 0.015 mol of
Q38: Which of the following aqueous mixtures would
Q52: Use a carefully drawn and labeled diagram
Q53: A 20.0-mL sample of 0.30 M HClO
Q57: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q58: The salts X(NO<sub>3</sub>)<sub>2 </sub>and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q59: A 25.0-mL sample of 0.35 M HCOOH
Q61: Use the following information to calculate the
Q89: What is the pK<sub>a</sub> for the acid