Multiple Choice
Nitric oxide reacts with chlorine to form NOCl. The data refer to 298 K. 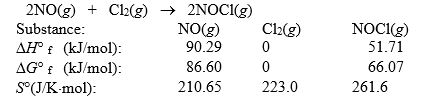 What is the value of G° for this reaction at 550 K?
What is the value of G° for this reaction at 550 K?
A) -143.76 kJ
B) -78.78 kJ
C) -22.24 kJ
D) -10.56 kJ
E) 66600 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which of the following should have the
Q12: As a chemical reaction proceeds toward equilibrium,
Q81: For a chemical reaction to be spontaneous
Q82: For a reaction at equilibrium, <font face="symbol"></font>S<sub>univ</sub>
Q83: Which of the following is true for
Q85: Given: C<sub>2</sub>H<sub>2</sub>(g) <font face="symbol"></font> 2C(graphite) + H<sub>2</sub>(g)
Q87: A reaction has a positive value of
Q88: Calculate <font face="symbol"></font>S° for the combustion of
Q89: Which relationship or statement best describes <font
Q90: Which of the following is always true