Multiple Choice
Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 
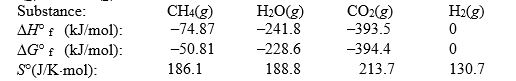
A) 8.2 × 1019
B) 0.96
C) 0.58
D) 1.2 × 10-20
E) 1.4 × 10-46
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: The term microstate refers to the energy
Q17: Consider the following quantities used in thermodynamics:
Q44: When a sky diver free-falls through the
Q48: Which of the following pairs has the
Q62: A certain process has <font face="symbol"></font>S<sub>univ</sub> >
Q63: Which of the following results in a
Q65: "A diamond is forever" is one of
Q67: Which relationship or statement best describes <font
Q68: For a process with <font face="symbol"></font>S <
Q69: Which relationship or statement best describes <font