Multiple Choice
The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K. 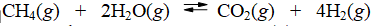
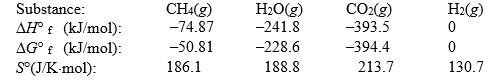
A) 658 K
B) 683 K
C) 955 K
D) 1047 K
E) 1229 K
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Consider the reaction <br>11ec6d53_97e1_49fd_9cd1_030bf8888a28_TB5833_<br>If the concentrations of
Q42: In the expression, S = k ln
Q43: Which of the following is true for
Q45: Which relationship best describes <font face="symbol"></font>S° for
Q47: Which relationship or statement best describes <font
Q48: Consider the figure which shows <font face="symbol"></font>G°
Q49: For what signs of <font face="symbol"></font>H and
Q50: Use the given data at 298 K
Q51: In order for a process to be
Q55: In order for a process to be