Multiple Choice
Calculate E°cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. 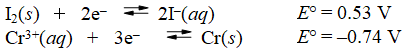
reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I-(aq)
A) E°cell = -1.27 V, spontaneous
B) E°cell = -1.27 V, nonspontaneous
C) E°cell = 1.27 V, spontaneous
D) E°cell = 1.27 V, nonspontaneous
E) E°cell = 1.54 V, spontaneous
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Two cells are connected in series, so
Q14: When the following redox equation is balanced
Q17: What is the value of the equilibrium
Q18: Which of the following statements about voltaic
Q21: A concentration cell is based on the
Q24: A battery that cannot be recharged is
Q34: Electrolytic cells utilize electrical energy to drive
Q37: Which of the following elements could be
Q77: Which one of the following pairs of
Q85: The lead-acid battery is an example of