Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 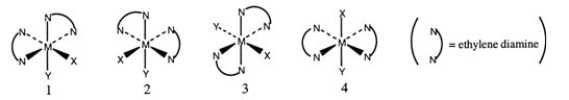 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Which one of the following has the
Q15: In the presence of a strong octahedral
Q17: The crystal field splitting energy, <font face="symbol"></font>,<br>A)
Q17: A certain transition metal complex has the
Q23: a. State the requirement for two molecules
Q29: What is the coordination number of cobalt
Q62: A feature of transition metal chemistry is
Q64: Which of the following ions is least
Q74: The most common oxidation state for ions
Q80: Square planar complexes can exhibit both geometric