Multiple Choice
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar) . 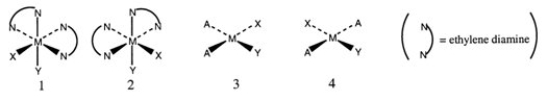 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) 1 and 2 are superimposable.
B) 1 and 2 are geometric isomers.
C) 3 and 4 are structural isomers.
D) 3 and 4 are optical isomers.
E) 3 and 4 are geometric isomers.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: A certain transition element has the stable
Q37: Which of the following transition elements can
Q40: What geometry is particularly common for complexes
Q44: If M represents a transition element, which
Q46: The compound K<sub>3</sub>[Fe(CN)<sub>6</sub>] is used in calico
Q51: Which of the following will be paramagnetic?<br>A)V
Q59: Which of the following ions could exist
Q68: Which of the following elements has the
Q69: Valence Bond theory rationalizes octahedral geometry by
Q75: The ground state electronic configuration of Cr<sup>2+</sup>