Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) 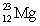
B) 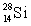
C) 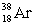
D) 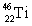
E) 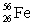
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: Identify the missing species in the following
Q47: Which one of the following equations correctly
Q48: Identify the missing species in the following
Q50: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg" alt="The isotope
Q53: What features do the r- and s-processes
Q55: Assuming that no other particles are produced,
Q55: Bombardment of uranium-238 nuclei by carbon-12 nuclei
Q56: Select the nuclide that completes the following
Q62: Detection of radiation by a Geiger-Müller counter
Q70: Who discovered radioactivity?<br>A)Geiger<br>B)Curie<br>C)Roentgen<br>D)Becquerel<br>E)Rutherford