Essay
The masses of a potassium-40 atom, a proton, and a neutron are 39.963999 amu, 1.007825 amu, and 1.008665 amu, respectively. Calculate to four significant figures
a. the mass defect in amu, and
b. the energy released in MeV/nucleon, in the formation of 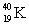 from the appropriate number of protons and neutrons.
from the appropriate number of protons and neutrons.
Correct Answer:

Verified
a. 0.3666 ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q72: So-called "magic numbers" of particles are thought
Q83: Which one of the following equations correctly
Q84: Briefly, explain the relationship between the rad
Q85: The nuclide Pb-210 undergoes three successive decays
Q86: Identify the missing species in the following
Q89: Write a complete, balanced equation to represent
Q90: An isotope with a high value of
Q91: Write a complete, balanced equation to represent
Q92: An 85-kg person exposed to barium-141 receives
Q93: Select the nuclide that completes the following