Multiple Choice
The specific heat capacity c of a metal is approximately related to its molar mass 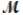 as follows: c ×
as follows: c × 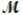 = 3R, where R is the universal gas constant, 8.314 J/molK. Use this relationship to identify the metal which has a specific heat capacity of 0.900 J/gK.
= 3R, where R is the universal gas constant, 8.314 J/molK. Use this relationship to identify the metal which has a specific heat capacity of 0.900 J/gK.
A) Li
B) Sn
C) Ca
D) Al
E) U
Correct Answer:

Verified
Correct Answer:
Verified
Q3: What is the final temperature when 20.0
Q59: Which one of the following is not
Q60: Calculate the <font face="symbol"></font>H°<sub>rxn</sub> for the decomposition
Q61: A system which undergoes an adiabatic change
Q62: Nitric acid, which is among the top
Q64: In which of the following processes is
Q65: The Starship Enterprise is caught in a
Q66: In a phase change of water between
Q67: The compound carbon suboxide, C<sub>3</sub>O<sub>2</sub>, is a
Q68: When 1.00 g of solid NH<sub>4</sub>Cl is