Multiple Choice
Which one of the following is a correct formation reaction?
A) C(diamond) C(graphite)
B) H2(g) + O(g) H2O(l)
C) C(graphite) + 4H(g) CH4(g)
D) 6C(graphite) + 6H2O(s) C6H12O6(s)
E) 2C(graphite) + 3H2(g) + 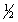 O2(g) C2H5OH(l)
O2(g) C2H5OH(l)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: For all processes, both q and w
Q12: In a reaction with high energy reactants
Q23: A system receives 575 J of heat
Q26: A system that does no work but
Q27: Cold packs, whose temperatures are lowered when
Q27: Which one of the following statements about
Q28: Calculate q when 28.6 g of water
Q29: When Karl Kaveman adds chilled grog to
Q32: a. Define, or explain fully what is
Q33: An ideal gas (the system) is contained