Short Answer
Given the following data: 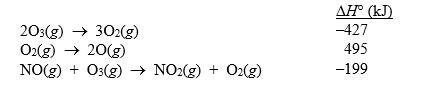 calculate H° for the reaction
calculate H° for the reaction
NO(g) + O(g) NO2(g)
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
F...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q1: If, as a pioneer, you wished to
Q12: Ethylene glycol, used as a coolant in
Q75: <font face="symbol"></font>H does not depend on the
Q76: a. A gas sample absorbs 53 kJ
Q77: Diborane (B<sub>2</sub>H<sub>6</sub>) has been considered as a
Q78: A mass of 1.250 g of benzoic
Q79: Starting from equations relating pressure to force
Q82: A system delivers 1275 J of heat
Q83: Calculate the enthalpy change for the reaction
Q84: Although internal energy (E) is more fundamental