Multiple Choice
Which of the following compounds would be expected to be more soluble in hexane (C6H14) ? 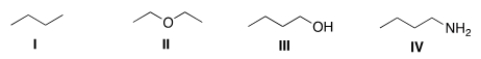
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What intermolecular force is generally considered the
Q25: Why do heteroatoms confer reactivity on a
Q28: Which of the following correctly matches the
Q29: Rank the following compounds in order of
Q30: The indicated bond is: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5871/.jpg" alt="The
Q31: Which of the following compounds has the
Q32: Which of the following intermolecular forces would
Q34: Which of the following molecules contain
Q35: Why do <font face="symbol"></font> bonds confer reactivity
Q38: Which of the following compounds can form