Multiple Choice
Determine the electron geometry around the indicated atom in each species. 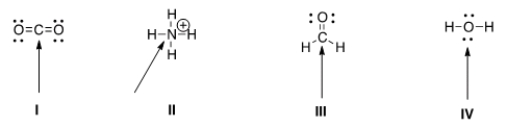
A) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
B) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear
C) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
D) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Q14: How many constitutional isomers are there for
Q25: Which compound contains the most polar
Q27: Which of the following molecules are
Q28: Which of the following Lewis structures is
Q29: Which molecule has the greatest difference in
Q31: Which of the following molecules contain
Q32: Which of the following is the appropriate
Q43: What is the hybridization of the nitrogen
Q66: What is the ground-state electronic configuration of
Q67: Which of the following molecules has polar