Multiple Choice
Refer to the figure below showing the chemical structures of several molecules. 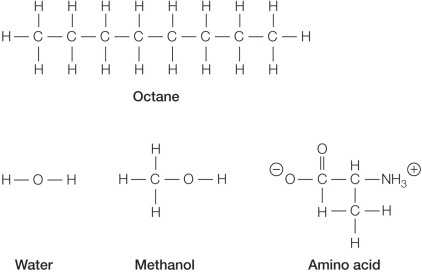 Which pair of molecules is most likely to be miscible (each soluble in the other) ?
Which pair of molecules is most likely to be miscible (each soluble in the other) ?
A) Octane and water
B) Water and methanol
C) Amino acid and octane
D) Methanol and octane
E) Amino acid and methanol
Correct Answer:

Verified
Correct Answer:
Verified
Q79: What type of chemical bond connects the
Q80: Refer to the chemical equation below.<br>2 H<sub>2</sub>O
Q81: Two carbon atoms held together in a
Q82: The subatomic particles that make up the
Q83: Refer to the balanced chemical equation below.
Q85: The equation C<sub>4</sub>H<sub>10</sub> + 7 O<sub>2 </sub><font
Q86: Differences in the electronegativity of atoms that
Q87: Carbon-14 is a radioactive isotope of carbon.When
Q88: Oxygen and carbon are defined as different
Q89: The two covalent bonds in a water