Multiple Choice
Refer to the figure below. 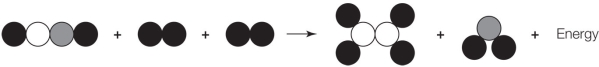 Which statement about the figure is true?
Which statement about the figure is true?
A) It shows a chemical change because the products differ from the reactants.
B) It shows a chemical change because the three molecules were transformed into two molecules.
C) It shows a chemical change because energy was released as a result of the change.
D) It does not accurately show a chemical change because the numbers of atoms on the two sides of the arrow differ.
E) It does not accurately show a chemical change because energy is shown on the wrong side of the arrow.
Correct Answer:

Verified
Correct Answer:
Verified
Q162: Which shows the elements carbon (C), hydrogen
Q163: Polar molecules<br>A) have electric charges that are
Q164: How does the ionic compound magnesium chloride
Q165: Ice floats because the ice crystals<br>A) contain
Q166: All of the elements listed below follow
Q168: Refer to the figure below showing various
Q169: A car sitting in the sun on
Q170: The ball-and-stick structure of methane (CH<sub>4</sub>) shows
Q171: Which statement is true?<br>A) Carbon makes the
Q172: How do the densities of ice and