Multiple Choice
Refer to the figures below. 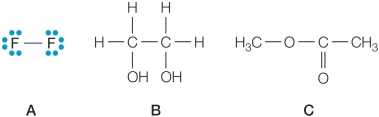 Which compound would have a higher heat of vaporization than water, and why?
Which compound would have a higher heat of vaporization than water, and why?
A) Compound A because it is smaller in size than water.
B) Compound A because unlike water, it is not capable of hydrogen bonding.
C) Compound B because it can form more hydrogen bonds per molecule than water.
D) Compound B because it contains more covalent bonds per molecule than water.
E) Compound C because it contains more oxygen atoms per molecule than water.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Hydrocarbons are _ and _, whereas salts
Q7: Which statement about chemical reactions is false?<br>A)
Q8: Some reactions, such as the decomposition of
Q9: The electronegativity of an atom is a
Q10: The mass of a proton serves as
Q12: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q13: Of the statements below, which best explains
Q14: Which pair has similar chemical properties?<br>A) <sup>12</sup>C
Q15: To determine the number of molecules in
Q16: The number of protons in a neutral