Multiple Choice
Refer to the figure below. 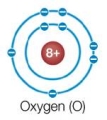 An oxygen atom by itself is very reactive, whereas an oxygen atom that has combined with two hydrogen atoms to form a water molecule is very stable.What explains this observation?
An oxygen atom by itself is very reactive, whereas an oxygen atom that has combined with two hydrogen atoms to form a water molecule is very stable.What explains this observation?
A) Oxygen starts out with a total of eight electrons and becomes more stable when it adds more electrons.
B) The eight protons in the oxygen nucleus need additional electrons from hydrogen atoms to help neutralize their charge.
C) The two electrons in the innermost shell of an oxygen atom need two additional electrons to balance out.
D) The mass provided by the two hydrogen atoms adds to the mass of the oxygen atom, which increases the stability of the entire structure.
E) An oxygen atom only has six electrons in its valence shell and needs two electrons from hydrogen to fill this shell.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: The best reference source for the atomic
Q34: Prolactin is a peptide hormone produced by
Q35: Isotopic analysis of biological samples can be
Q36: The five statements below describe properties of
Q37: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q39: The ability of an atom to combine
Q40: An atom has 36 protons and 44
Q41: One mole of ADP undergoes a condensation
Q42: The Australian environmental protection agency defines bioremediation
Q43: Which statement provides evidence that living organisms