Multiple Choice
Refer to the figure below. 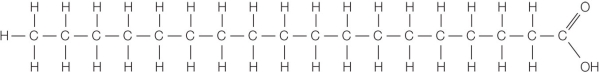 The figure shows a molecule found in biological samples.What interactions will occur if 1 part of this compound is mixed with 20 parts of water, and why?
The figure shows a molecule found in biological samples.What interactions will occur if 1 part of this compound is mixed with 20 parts of water, and why?
A) Hydrophobic interactions will cause these molecules to disperse evenly between the water molecules.
B) Hydrophilic interactions will cause these molecules to disperse evenly between the water molecules.
C) Hydrophobic interactions between these molecules and water will cause all of the molecules to form a separate layer.
D) Both hydrophobic and hydrophilic interactions between these molecules and water will cause groups of the molecules to form small spherical bunches dispersed in the water.
E) Both hydrophobic and hydrophilic interactions between these molecules and water will cause single molecules to disperse evenly between the water molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q109: Which general chemical principles can be applied
Q110: _ capacity is a term used to
Q111: In the periodic table, why are hydrogen,
Q112: Surface tension and cohesion occur in pure
Q113: Carbon-12 is the most abundant isotope of
Q115: If the pH of an acid rain
Q116: When 0.1 mole of sodium hydroxide (NaOH)
Q117: What features of the water molecule are
Q118: How would you make 100 mL of
Q119: Refer to the oxidation-reduction reaction below. <br>Fe