Multiple Choice
Refer to the table below. 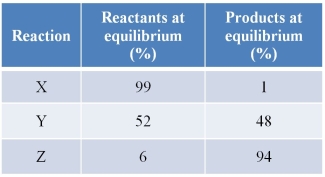 How do you predict the G° values for these reactions will compare?
How do you predict the G° values for these reactions will compare?
A) All of the reactions will have positive G° values, with Z lowest and X highest.
B) All of the reactions will have negative G° values, with Z having the least negative and X the greatest negative value.
C) Y will have the smallest G° value, while X will have a large positive G° and Z will have a large negative G°.
D) Y will have the smallest G° value, while X will have a large negative G° and Z will have a large positive G°.
E) Y will have the largest G° value, while X will have a small positive G° and Z will have a small negative G°.
Correct Answer:

Verified
Correct Answer:
Verified
Q141: Enzyme Y is a component of a
Q142: The conversion of A to B, which
Q143: A chemical reaction is found to have
Q144: How does the second law of thermodynamics
Q145: Coenzymes differ from enzymes in that coenzymes
Q147: Enzymatic reactions can become saturated as substrate
Q148: Fireflies<br>A) release a considerable amount of energy
Q149: Which of the following represents kinetic energy?<br>A)
Q150: Which reaction will go the furthest toward
Q151: Which of the following is neither created