Multiple Choice
Refer to the figure below. 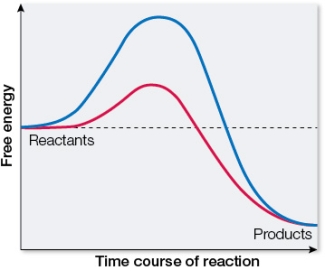 This graph shows the change in free energy for the same reaction with and without a catalyst.Which tracing represents the catalyzed reaction and why?
This graph shows the change in free energy for the same reaction with and without a catalyst.Which tracing represents the catalyzed reaction and why?
A) The blue tracing represents the catalyzed reaction because it has a larger G than the uncatalyzed reaction.
B) The blue tracing represents the catalyzed reaction because it has a larger activation energy than the uncatalyzed reaction.
C) The red tracing represents the catalyzed reaction because it has a smaller G than the uncatalyzed reaction.
D) The red tracing represents the catalyzed reaction because it has a smaller activation energy than the uncatalyzed reaction.
E) The red tracing represents the catalyzed reaction because it leads to the formation of more products than the uncatalyzed reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q220: Certain types of cells have very low
Q221: Which is an example of an enzyme
Q222: Which series best summarizes the relative energies
Q223: What is the order in which the
Q224: Which of the following could we infer
Q226: In which sequence does the form of
Q227: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q228: How do competitive and noncompetitive enzyme inhibitors
Q229: The addition of the competitive inhibitor mevinolin
Q230: The phosphorylation of glucose to glucose 6-phosphate