Multiple Choice
Refer to the figure below. 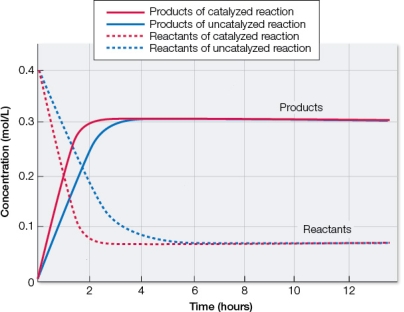 Based on the graph, which statement best describes these chemical reactions?
Based on the graph, which statement best describes these chemical reactions?
A) Addition of a catalyst to a reaction does not affect the equilibrium position of the reaction, but only increases the rate of attainment of equilibrium.
B) Addition of a catalyst to a reaction speeds up the reaction by decreasing the amount of energy input needed to get over the activation energy barrier.
C) Addition of heat to a reaction speeds up the reaction by increasing the kinetic energy of reactant molecules, which increases collisions that result in products.
D) Addition of energy must be made to exergonic reactions in order to allow reactants to achieve the transition state that can then proceed spontaneously to form products.
E) Addition of energy to an endergonic reaction above that needed to go from reactants to products must be made in order to allow reactants to achieve the transition state.
Correct Answer:

Verified
Correct Answer:
Verified
Q79: Hexokinase is the enzyme that catalyzes the
Q80: Suppose the reaction A <font face="symbol"></font> B
Q81: What features of an enzyme's active site
Q82: It is estimated that approximately 90 percent
Q83: Denatured enzymes are<br>A) ribozymes.<br>B) synthesized in vitro.<br>C)
Q85: Refer to the figures below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q86: In solution, ATP is readily hydrolyzed into<br>A)
Q87: An inhibitor that binds reversibly to the
Q88: Which are biological examples of potential energy
Q89: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"