Multiple Choice
Refer to the figure below, which shows an oxidation-reduction reaction that occurs spontaneously. 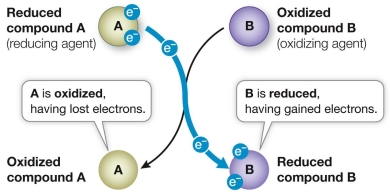 Which statement correctly compares the free energy stored in molecules shown in the figure?
Which statement correctly compares the free energy stored in molecules shown in the figure?
A) The reduced form of B has greater free energy than the reduced form of A.
B) The oxidized form of B has greater free energy than the reduced form of A.
C) The reduced form of A has greater free energy than the oxidized form of A.
D) The oxidized form of B has greater free energy than the reduced form of B.
E) The oxidized form of A has greater free energy than the reduced form of B.
Correct Answer:

Verified
Correct Answer:
Verified
Q138: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q139: Refer to the table below, which summarizes
Q140: The formation of glucose from glycolytic and
Q141: Like glycolysis, fermentation occurs in the<br>A) mitochondria.<br>B)
Q142: Which statement about metabolic pathways is true?<br>A)
Q144: Which statement best describes steps 1, 2,
Q145: Refer to the figure below, showing inputs
Q146: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q147: Under anaerobic conditions, pyruvate<br>A) is converted to
Q148: The main difference between lactic acid fermentation